Serialisation
Simplifying a complex subject to deliver product insight and value
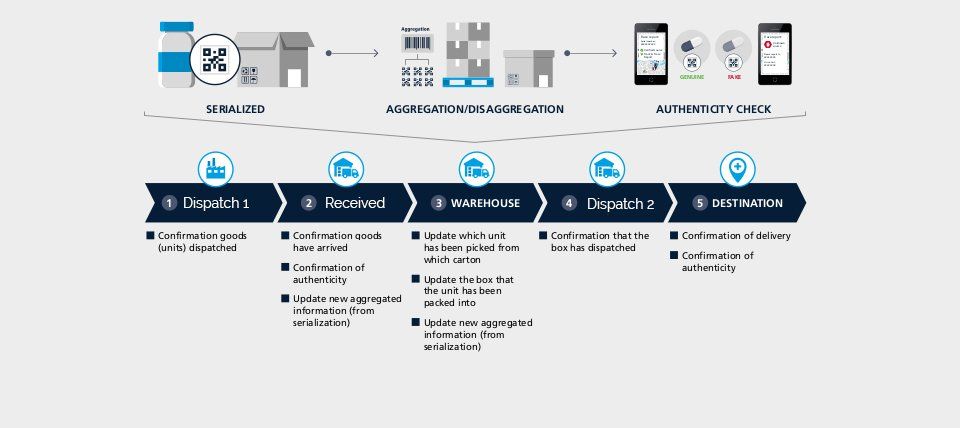
Serialisation is a powerful tool that can transform a large range of functions in an organisation from marketing through supply chain to product security. All the projects that have been completed have delivered clear business case and return on investment.
However, many organisations underestimate the impact and the potential disruption that can occur when implementing a serialisation solution. These include:
• Manufacturing
• Regulatory & Compliance
• IT including ERP/MES Systems
• Supply chain
• Sales & Brand management
Our mission is to deliver process understanding, expertise and cross functional knowledge to reduce complexity and enhance the product and brand value to deliver increased efficiency and profitability